Application Functionality
Link to the application: https://appsrk.aotm.gov.pl/
The SRK application is a free, innovative tool for healthcare entities and enables cost accounting, pricing of medical procedures, benchmarking, and effective management.
Application modules:
- data entry
- pricing of medical procedures,
- reports,
- management dashboard,
- indicators.

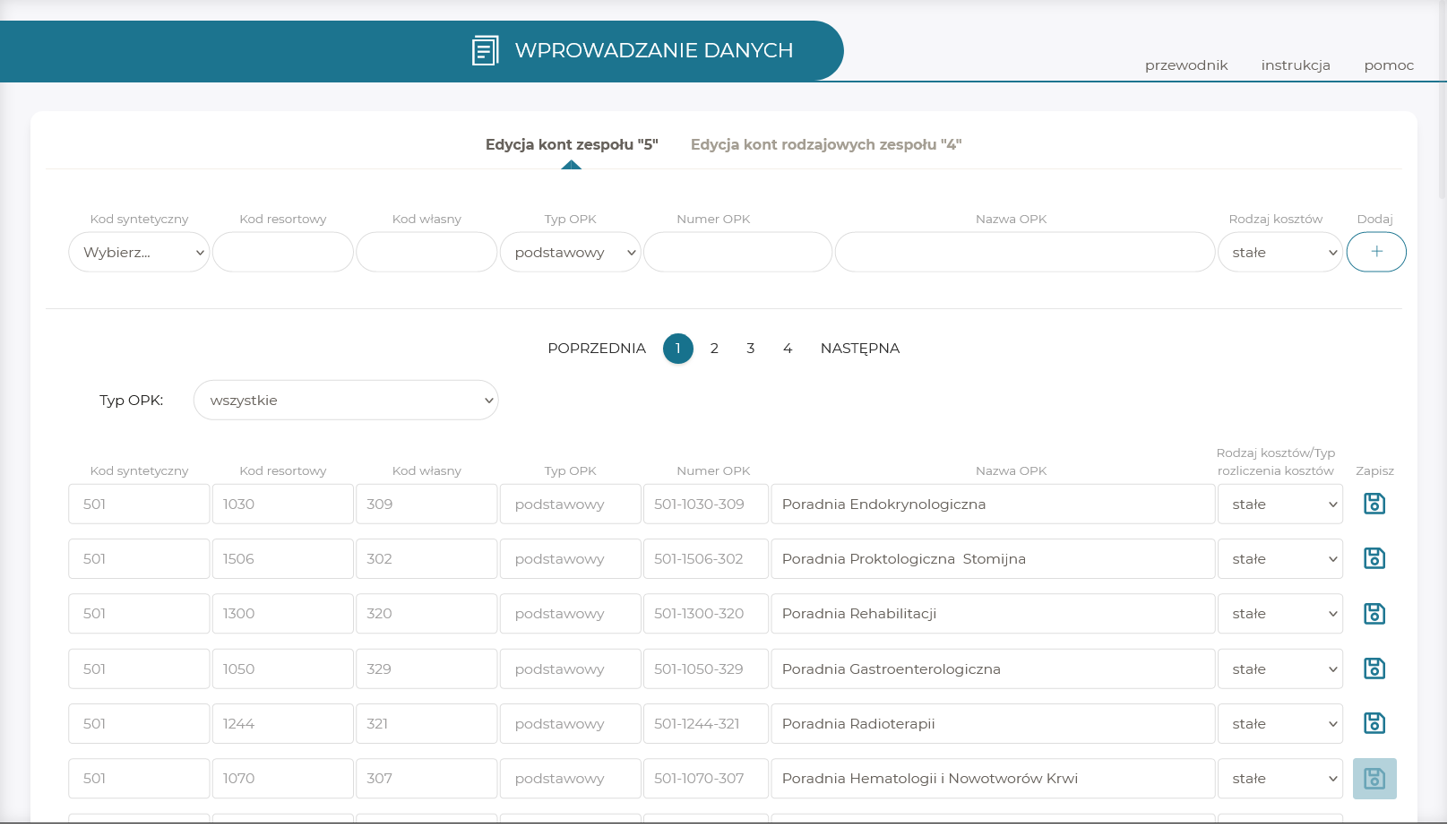
Data entry:
- data import to the application from an MS Excel file in line with a prepared template,
- possibility to enter or edit the data directly in the system,
- cost accounting in compliance with the Regulation of the Minister of Health of 26 October 2020 on recommendations regarding the cost accounting standard at healthcare providers.
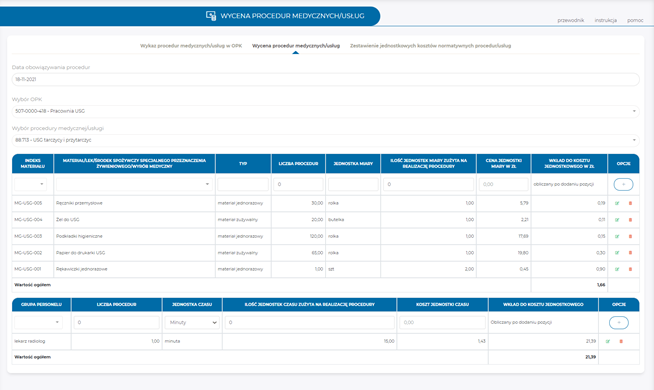
Pricing of medical procedures
- import of normative unit costs of medical procedures using an MS Excel file template when the pricing is performed outside the application,
- import of dictionaries for prices of direct materials and healthcare personnel rates,
- pricing of normative unit costs of medical procedures,
- an easy way to periodically update the pricing of medical procedures.
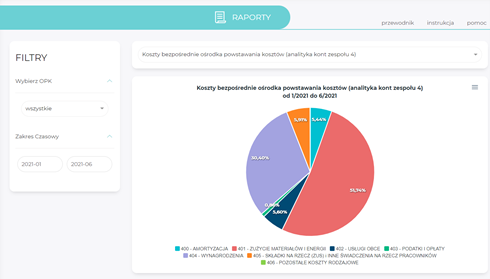
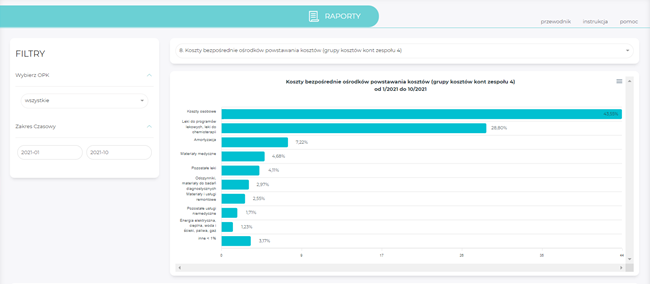
Reports
- preparation of reports containing the most relevant financial information,
- display of sorted data per selected filters: type of activity, type of cost centre, time range,
- presentation of data entered in the form of simple tables (with the option to expand the data to the analytical level) and charts,
- option to download charts and tables to CSV, SVG, PNG/DOC or XLSX files,
- option to expand the reporting base according to the needs of the healthcare providers.
Management dashboard
- simultaneous presentation of data from multiple sources,
- general-to-specific multidimensional data analysis,
- support in management’s decision-making.
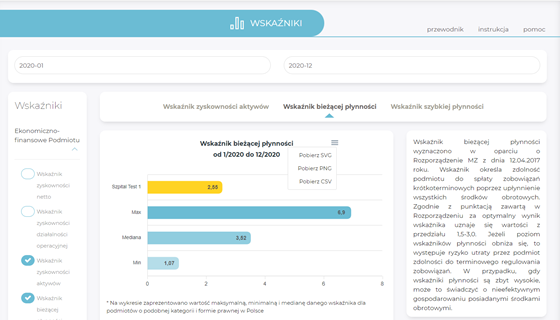
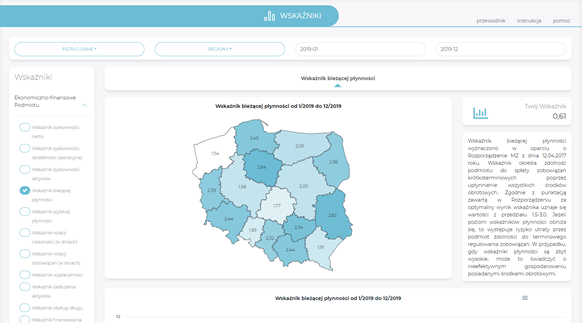
Indicators
- access to information on basic values of economic and financial indicators and statistical indicators at the level of the entity and individual cost centres,
- comparison of one’s own results with those of other domestic entities or other entities in individual provinces (anonymised data).
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2024]