Off-label
In Poland, medicines may also be reimbursed outside the indications included in their marketing authorisation (listed in the Summary of Product Characteristics). In such cases, the Agency for Health Technology Assessment and Tariff System participates in the reimbursement procedure.
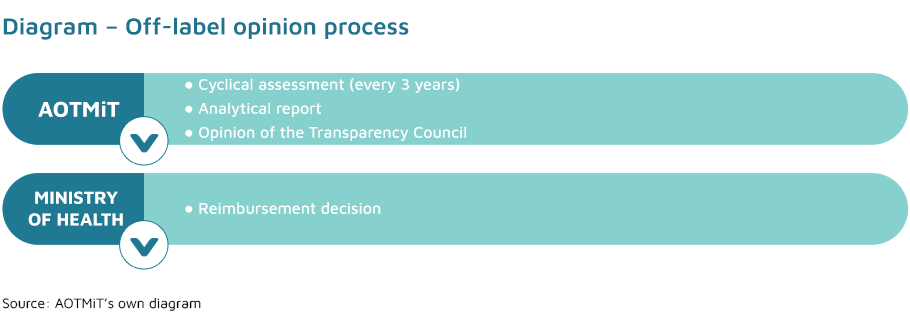
After consulting the Transparency Council and the national consultant in the relevant branch of medicine the Minister of Health may issue (ex officio and based on relevant clinical data) an administrative decision on the reimbursement of a medicine in an indication, dosage or route of administration different from those specified in the Summary of Product Characteristics (the so-called off-label use) within the meaning of the Act of 6 September 2001– Pharmaceutical law.
The Transparency Council takes into account:
- the medicine’s efficacy and effectiveness;
- its safety of use;
- the relationship between health benefits and risks of use;
- the impact on the expenditure of both the National Health Fund and the patients;
- the existence of alternative health technologies and their efficacy and safety of use;
- health priorities and cost-effectiveness ratio;
- the threshold for the cost of obtaining an additional quality-adjusted life year.
The Transparency Council issues an opinion on the reimbursement of a technology in an off-label indication within 14 days, taking into account, in particular, the severity of the clinical condition in which the medicine is to be used. The opinions of the Transparency Council in question are binding and valid for a period of three years. Within 30 days before the expiry of the previous opinion, the Transparency Council and the national consultant in the relevant branch of medicine, issue a subsequent opinion, in respect of the particular active substance in the indication in question, unless:
- the Minister of Health provides the President of the Agency or a national consultant in the relevant branch of medicine with information on his/her intention not to continue reimbursement in the indication in question, or
- the indication in question is to be included in the Summary of Product Characteristics.
The President of the Agency publishes the aforementioned opinion of the Transparency Council in the Agency’s Public Information Bulletin, with an indication of its expiry date, along with the working material on which it is based.
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2024]

