Reform
Reform of the health benefit package
Analytical activities carried out as part of the process of updating the health technologies constituting the health benefit package include the development of assumptions for new systemic solutions in the organisation of the provision of guaranteed healthcare services with the group of healthcare system stakeholders.
The aim of the undertaken activities is to develop (on the basis of experience/mechanisms applied in other countries) a new model of organisation of diagnostics and treatment, within the framework of particular reference levels of the healthcare system, conducted in a coordinated and comprehensive manner, in order to achieve a health effect and comfort of life for patients. These actions constitute a response to the patients’ expectations with regard to improvement of the quality and effectiveness of the diagnostic and therapeutic process. It is also anticipated that the undertaken actions will optimise the costs of therapy and change the allocation of financial resources within the healthcare system.
As part of the new systemic solutions, changes in the organisation of the provision of healthcare services are being developed, e.g. in the area of therapeutic rehabilitation, oncology, haematology, to ensure shortening the time of waiting for the diagnosis/start of appropriate therapy, including coordination of diagnostic and therapeutic processes.
The concept of organisational changes in the field of coordinated and comprehensive care includes the definition of the basic defining elements:
- the right of beneficiaries to receive a given healthcare service;
- the scope and profile of medical interventions;
- the detailed organisational conditions, including the conditions for the provision of healthcare services (in accordance with the adopted model of service description), aimed at adjusting the profile of provided interventions within particular healthcare services to the health problem.
The activities taken under the process also include the development (with the participation of experts) of:
- clinical guidelines;
- quality indicators for the diagnostic and therapeutic process (structure, processes and results);
- a set of tariffs for diagnostic, therapeutic and monitoring services.
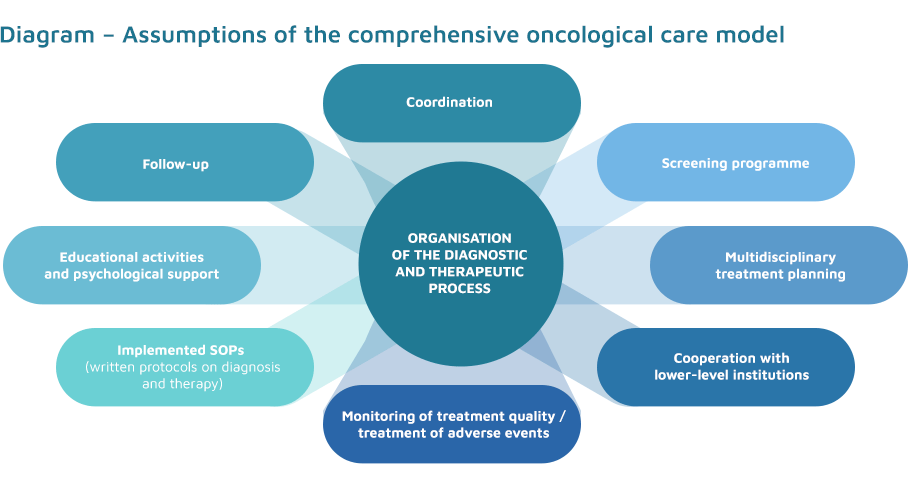
The model of a comprehensive approach to oncological diagnosis and treatment assumes:
- a coordinated diagnostic and therapeutic process;
- diagnostics and treatment in specialised centres;
- functioning of a multidisciplinary team;
- follow-up after treatment is completed;
- prospective monitoring of treatment quality.
Analytical work on the implementation of the Minister of Health’s commissions on the preparation of concepts of new organisational solutions for coordinated and comprehensive care, similarly as in the case of the process of reforming the health benefit package, requires cross-cutting actions, which take into account the preparation of three basic elements of comprehensive oncological care, i.e.:
- recommendations for diagnostic and therapeutic procedures;
- definition of the necessary standard in terms of organisational conditions and diagnostic and therapeutic processes for each “competence centre”, based on clinical management standards, including quality requirements and cooperation frameworks within the healthcare system ensuring the uninterrupted diagnostic and therapeutic chain;
- measures to assess the quality of oncological diagnostics and treatment, prepared on the basis of clinical management recommendations.
Due to the need to carry out complex analyses in this process and to involve several analytical teams (HTA analysts, healthcare services analysts, data analysts, BI analysts, statistics) and clinical and systemic experts at the same time, analytical works related to the preparation of analytical materials on the development of forms of cancer patient care takes nearly 6 months. The developed analytical study with the proposed concept of oncological care organisation is then submitted to the Transparency Council for review.
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2024]

