Guidelines
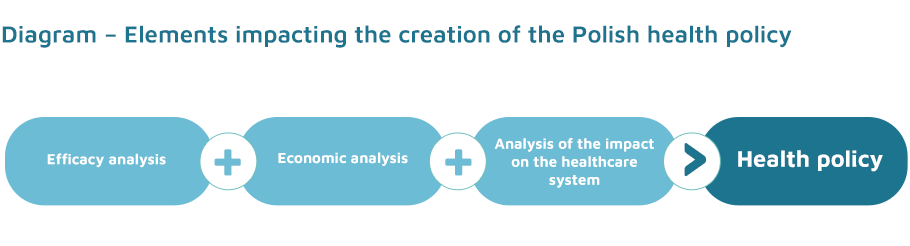
The health technology assessment guidelines constitute a set of information applied by analysts of the Agency for Health Technology Assessment. They are used to prepare transparent analyses summarising health, social, economic and ethical information (available also in other countries of the world) about a given health technology. In its work, the Agency depends on scientific evidence which demonstrates, for example, whether a medicine is safe and efficacious for the patient. Such information is essential when making decisions that shape the state’s health policy. The full assessment consists of 3 elements.
Health technology assessments are the basis for recommendations of the independent Transparency Council concerning the financing of medicines, foodstuffs for particular nutritional uses and medical devices, as well as healthcare services, from public funds.
Health technology assessment (HTA) consists in assessing the incremental benefits in terms of efficacy and safety, associated with the introduction of a new health technology into clinical practice in relation to the incremental costs associated with that introduction.
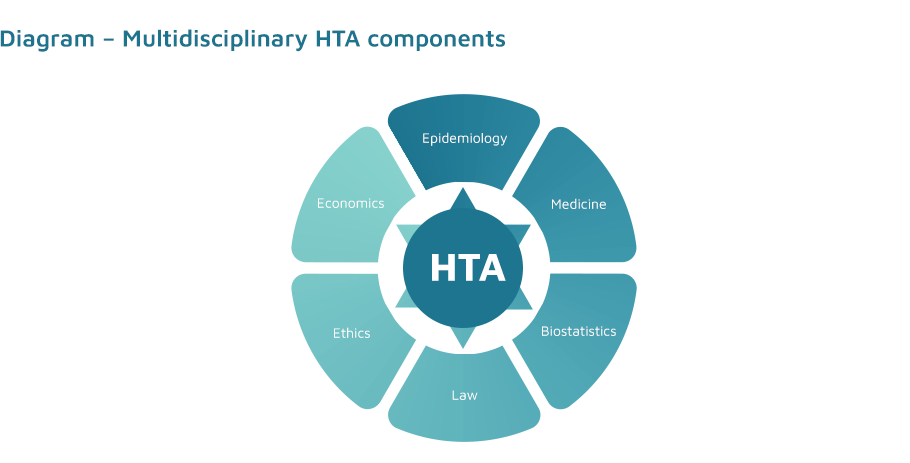
It is an interdisciplinary field of knowledge, the aim of which is to support decision makers in making evidence-based decisions on the reimbursement of innovative medicines and non-pharmaceutical health technologies. It is a process which aims to summarise the available information on health, economic, social and ethical aspects of the application of health technologies, conducted in a transparent and systematic manner, in accordance with commonly accepted principles (HTA guidelines), in order to obtain the highest possible reliability of results.
The full health technology assessment report (HTA report) consists of:
- decision problem analysis;
- clinical analysis;
- economic analysis;
- analysis of the impact on the healthcare system.
The initiation of a health technology assessment must be preceded by an analysis of the decision problem, which constitutes a specific protocol for the clinical, economic and financial analyses.
Version 3.0 of the Health technology assessment guidelines was developed in August 2016.
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2024]

