Adaptation process
AOTMiT has developed an accelerated pathway for guideline development, based on many years of experience in issuing opinions on recommendations for diagnostic and therapeutic pathways and methodological knowledge, which allows for their development when needed due to a threat to public health.
The guidelines under the accelerated pathway are developed by way of a cooperation of :
- clinical experts
- and Agency’s analysts.
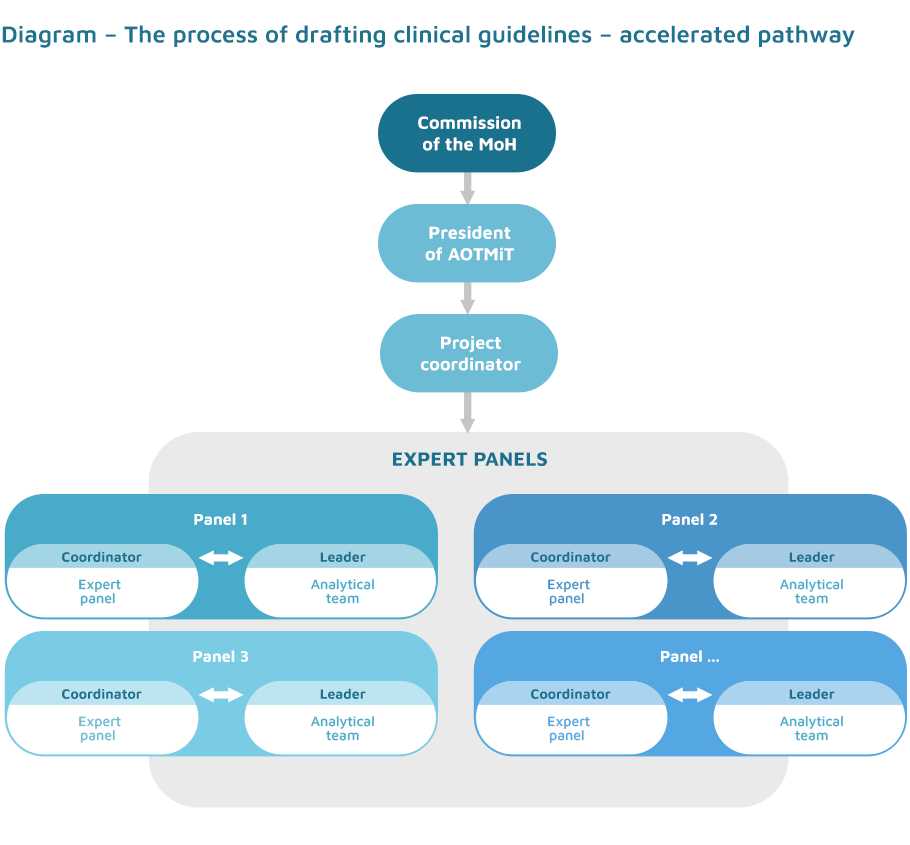
From a methodological point of view, the works are based on the ADAPTE methodology and use many elements of the guidelines’ adaptation process:
- definition of the detailed scope and formulation of appropriate clinical questions;
- creation of a recommendation matrix that compares global guidelines in a given area;
- detailed analysis of publications identified in the systematic reviews;
- development of the recommendations on the basis of collected data and scientific evidence;
- discussion on the acceptability and applicability of recommendations;
- final drafting of the recommendations.
Whenever the situation requires, after the publication of the first version of the recommendations, the teams carry out regular updating reviews in order to collect scientific information and evidence that may indicate a need for an update of the guideline document.
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2024]

