Recommendations
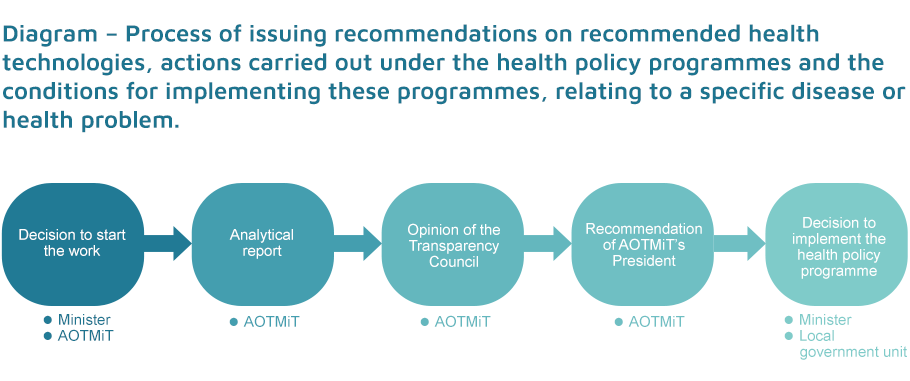
The Agency prepares recommendations which specify recommended health technologies, actions and conditions for the implementation of health policy programmes concerning a given disease or a given health problem. The legal basis for the implementation of the above-mentioned activities is Article 48aa of the Act on healthcare services financed from public funds (Journal of Laws 2020, item 1398, as amended). According to the provisions of the Act, the Agency may prepare the said recommendations on its own initiative or upon a commission of the Minister of Health.
The general objective of the recommendation is to simplify the implementation of the Health Policy Programme (HPP) by local self-government units.
HPPs which have been prepared in accordance with the published recommendation of the President do not require the opinion of the President of the Agency. In such cases, the local self-government unit must send a statement to the Agency, confirming that the prepared draft HPP is compliant with the applicable recommendation. At the end of the HPP implementation, the local self-government is also obliged to send a report summarising the Programme implementation.
The analytical report is prepared in accordance with HTA (health technology assessment) and EBM (evidence-based medicine) principles, based on foreign and Polish literature. The creative process is based on the health technology assessment guidelines and the act of law applicable to the recommendations.
For the most recent recommendations, please visit the Public Information Bulletin page.
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2024]

