Healthcare services

Services may be provided by both public and non-public healthcare providers, as well as by medical practitioners or by a group practice of nurses and midwives. The form of ownership of the healthcare provider is not important, as all the entities should treat the patient equally.
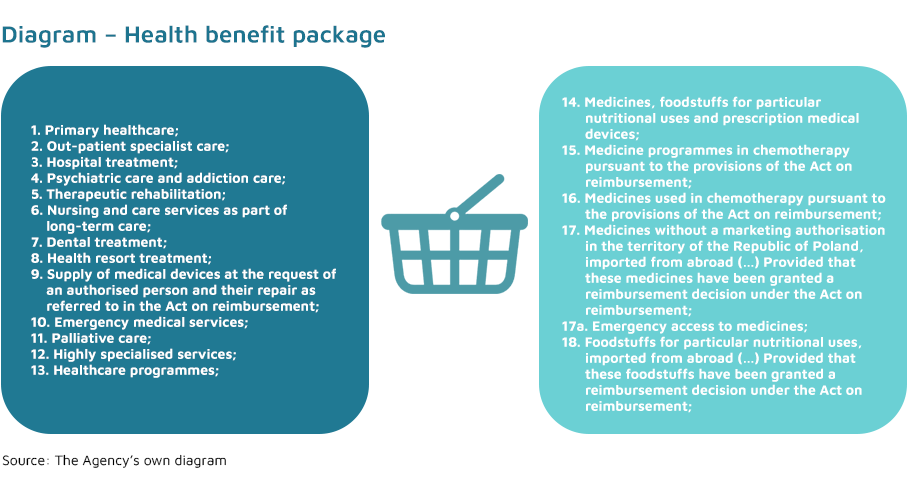
Health benefit package – these are healthcare services provided to persons entitled under the universal health insurance system. It includes: health services, health services in kind and associated services.
The range of health benefit package is defined in Article 15(2) of the Act of 27 August 2004 on healthcare services financed from public funds.
All works related to the assessment of healthcare services, including medical devices and guidelines for diagnostic and therapeutic procedures are carried out under commissions of the Minister of Health.
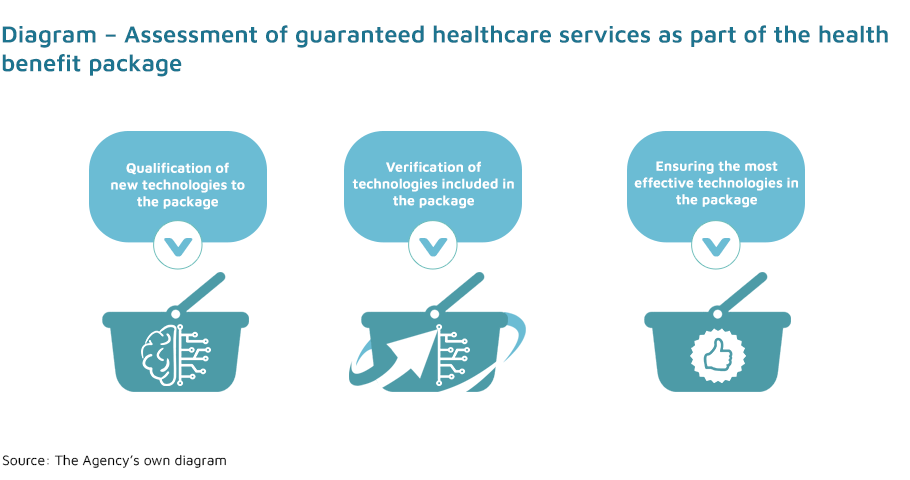
The Agency for Health Technology Assessment and Tariff System carries out statutory tasks in three areas:
- assessment of healthcare services (non-pharmaceutical technologies), in the process of: qualifying healthcare services for the health benefit package, removing healthcare services from the package, changing health technologies;
- reorganisation of the health benefit package;
- determining of practice guidelines by issuing opinions on clinical guidelines prepared by scientific societies.
The first two areas are implemented in line with EBM (evidence-based medicine) and HTA (health technology assessment) principles. The third area is based on the international guidelines AGREE II and ADAPTE, as well as the methodology developed in cooperation with experts for verifying and issuing opinions on draft guidelines and their adaptation in the healthcare system.
AOTMiT’s role in the development of the health benefit package
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2024]

