Qualification
Qualification of healthcare services into the health benefit package
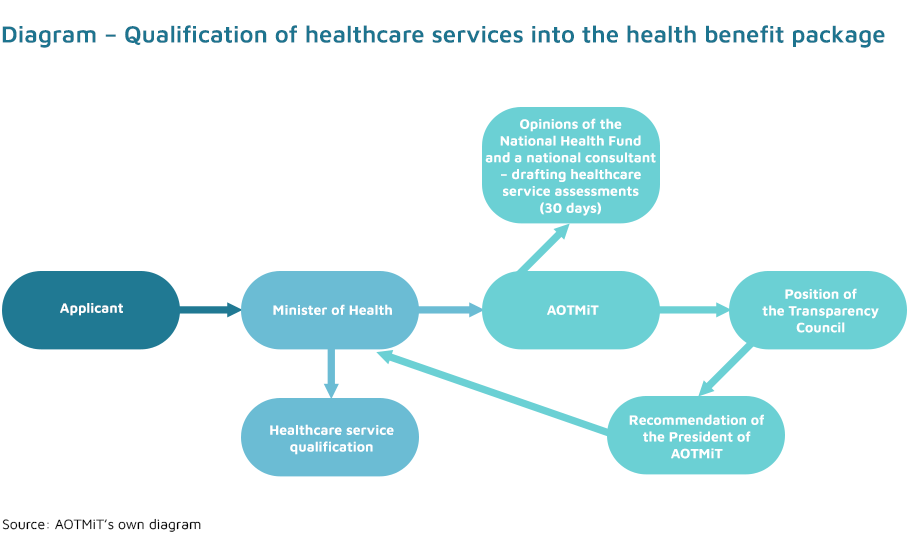
The initiative to qualify a new health technology into the health benefit package belongs exclusively to the Minister of Health (Article 31c (1) of the Act on healthcare services). Applications for the introduction of a new healthcare service may be submitted to the Minister of Health by all participants of the healthcare system – in practice, they are submitted by national consultants, scientific societies and patient organisations.
The assessment of healthcare services is conducted under a commission from the Minister of Health. The Minister’s commission provides a description of healthcare service, a disease or health condition in which the service is provided and information on the impact of the service on the improvement of citizens’ health.
The assessment of the healthcare service in question is carried out with regard to the criteria specified in Article 31a (1) of the Act on healthcare services. These criteria serve as an interpretation for a detailed analysis of the impact of specific healthcare services on the health of the society. Impact of the proposed healthcare service on the improvement of citizens’ health, health priorities and incidence, prevalence or mortality rates should all be considered. Health priorities are legally defined by the Minister of Health by means of a regulation, taking into account the citizens’ health condition and the need to obtain the best health effects.
The final decision on inclusion of a healthcare service into the health benefit package is made by the Minister of Health. Efficacy and effectiveness, safety of use, the relation of health benefits to the risk of use and the impact on the public payer and patients’ budgets all have to be taken into consideration. The criteria listed in Article 31a apply in all other cases.
Criteria for qualifying benefits for the guaranteed benefits package:
- impact on the improvement of citizens’ health, taking into account: health priorities specified in the regulations issued on the basis of para. 2, the incidence, morbidity or mortality rates determined on the basis of current medical knowledge;
- the effects of the consequences of an illness or health condition, in particular leading to: premature death, inability to live independently / inability to work within the meaning of the provisions on pensions from the Social Insurance Fund, chronic suffering or chronic illness, reduction of the quality of life;
- importance for the health of citizens, taking into account the need to rescue and achieve full recovery or improve health, prevent premature death, improve quality of life without significantly affecting its length;
- clinical efficacy and safety;
- the ratio of the health benefits obtained to the health risk;
- ratio of costs to achieved health effects;
- financial consequences for the health care system, including for entities obliged to finance health care services from public funds.
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2024]

