Vaccines
A vaccine is a biological preparation, which is to imitate a natural infection and leads to the development of immunity similar to that obtained by the body during the first contact with the real micro-organism (bacterium or virus).
The vaccine consists of one or more antigens which are obtained from living or killed micro-organisms, their purified fragments or metabolic products of bacteria as well as through genetic engineering. In addition, the vaccine may contain stabilisers and preservatives that protect against micro-organisms, substances that strengthen and speed up the occurrence of immunity, as well as traces of substances from the production process.
Live vaccines develop strong immunity after a single dose. However, inactivated (killed) vaccines require the administration of several doses. The antigen included in the vaccine injected into the body stimulates the immune system cells to produce specific antibodies. Immunological memory cells which provide a long-term protective effect of the vaccination are also formed. If immunological memory cells come into contact with the pathogenic micro-organism, they produce specific antibodies that prevent the disease from developing.
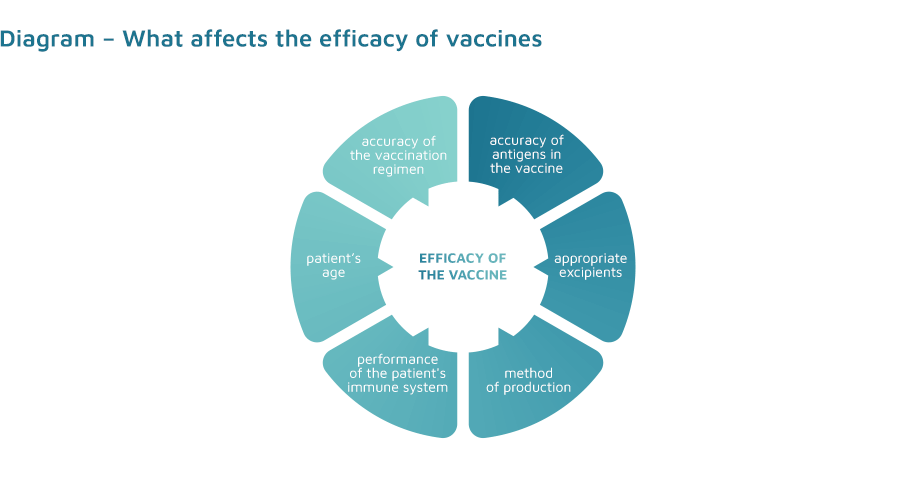
The efficacy of a vaccine depends on the use of appropriate vaccine antigens and appropriate excipients, mainly adjuvants, the method of production, the performance of the immune system of the vaccinated person and their age, as well as a properly selected vaccination regimen.
In accordance with Article 31 ca. 1 paragraph of the Act of 27 August 2004 on healthcare services financed from public funds (Journal of Laws of 2024, item 146), the Minister of Health may commission the President of the Agency to prepare recommendations concerning the justifiability of the use of medicines within the framework of The Preventive Vaccination Programme (PVP), referred to in the provisions on preventing and combating human infections and infectious diseases.
Upon receipt of the commission, the President of the Agency orders the marketing authorisation holder (within the meaning of the Act of 6 September 2001 – Pharmaceutical law), to present:
- a clinical analysis;
- an economic analysis;
- an analysis on the impact on the National Health Fund’s budget;
– referred to in Article 25(14)(c), first to third indent, of the Act on Reimbursement within 3 months of receiving the request.
The provisions of Article 31c (3)-(9) of the Act apply to the development of this recommendation. The recommendation is issued within 2 months from the date of receiving the analyses or from the expiry of the above-mentioned deadline. In the case of failure to provide the analyses, the President of the Agency issues a recommendation based on the available data.
In the case of assessments of preventive vaccination programmes, the Agency prepares analytical materials in accordance with the HTA guidelines, including an efficacy assessment, a cost-effectiveness assessment, a budget impact analysis, a review of the clinical practice guidelines and reimbursement recommendations, as well as expert opinions on the validity of using the health technology in question in a public vaccination carried out within the framework of the Preventive Vaccination Programme (PVP).
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2024]

